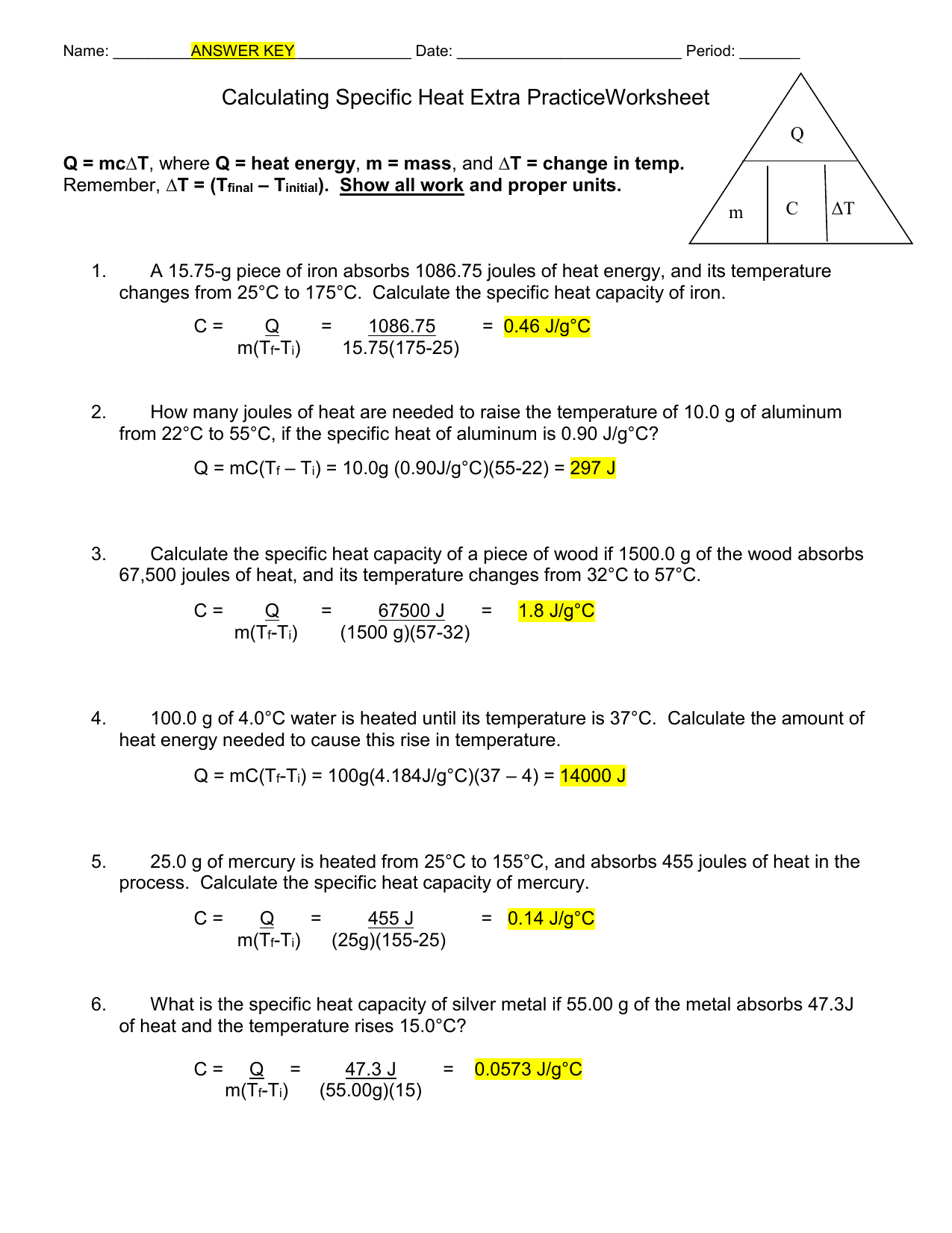
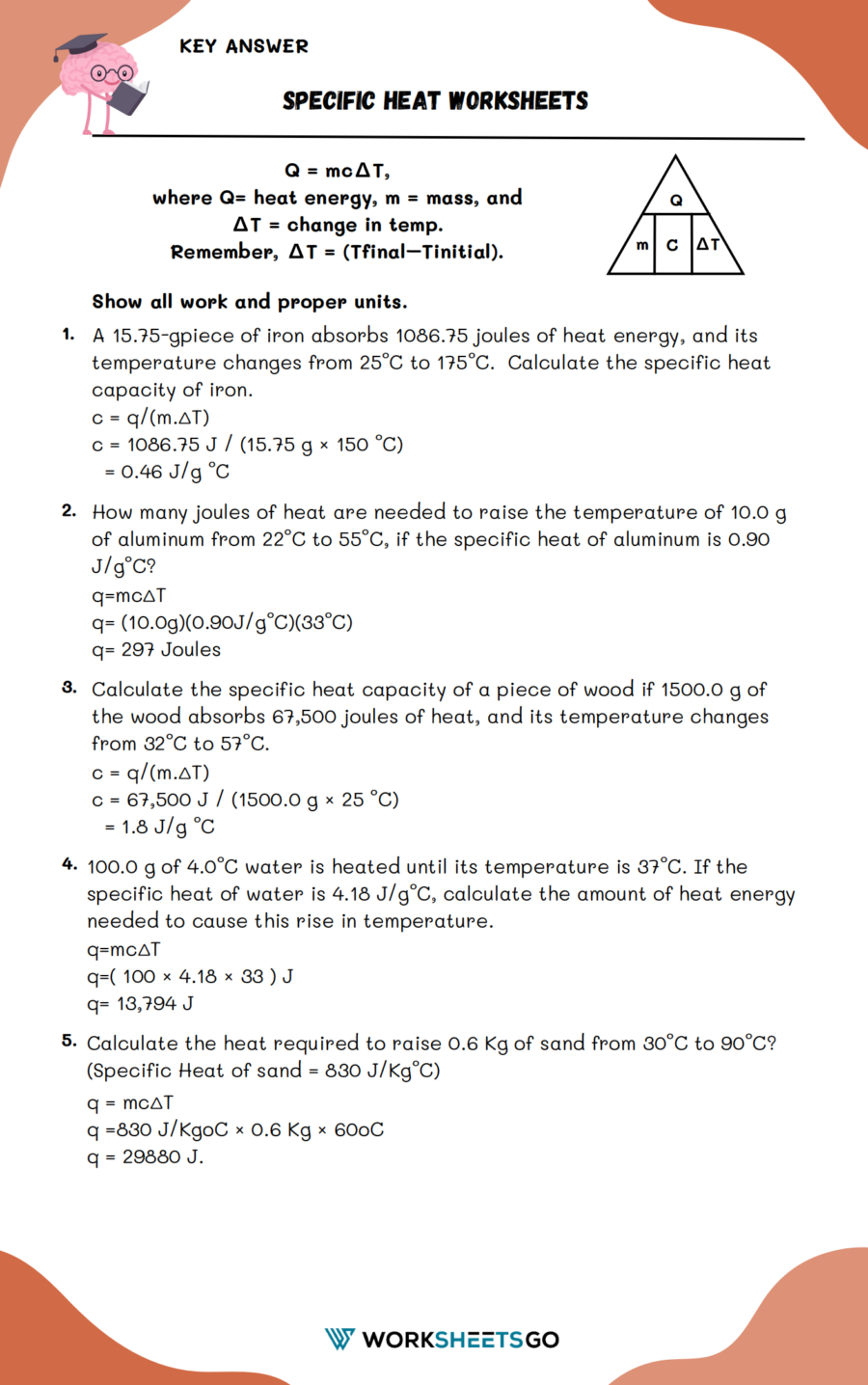
Specific Heat Worksheet Answers - Use q = (m)(δt)(cp) to solve the following problems. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil? Show all work and units. Show all work and proper. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the.
Show all work and proper. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. It requires more energy to raise its temperature by the same amount. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. What does it mean if water has a higher specific heat capacity than oil?
It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil? Use q = (m)(δt)(cp) to solve the following problems. Show all work and proper. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units.
Calculating Heat And Specific Heat Worksheets
It requires more energy to raise its temperature by the same amount. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. Show all work and units. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show.
Math Skills Specific Heat Worksheet Answers
Show all work and proper. Show all work and units. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil?
16 Specific Heat Worksheet /
What does it mean if water has a higher specific heat capacity than oil? Show all work and units. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. It requires more energy to raise its temperature by the same amount. Show all work and proper.
Specific Heat Capacity Worksheet With Answers
Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil?
Specific Heat Worksheet Answers 1
It requires more energy to raise its temperature by the same amount. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units. What does it mean if water has a higher specific heat capacity than oil? Answers to worksheet # 17 calculating heat the specific heat capacity.
40 specific heat capacity worksheet answers Worksheet Resource
Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. Use q = (m)(δt)(cp) to solve the following problems. Show all work and proper. It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific.
Specific Heat Calculations KEY Calculating Specific Heat
Show all work and proper. It requires more energy to raise its temperature by the same amount. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
Specific Heat Worksheet
It requires more energy to raise its temperature by the same amount. Use q = (m)(δt)(cp) to solve the following problems. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. Show all work and units. What does it mean if water has a higher specific.
specific heat worksheet answers 1 Worksheet Zone
Show all work and proper. Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. What does it mean if water has a higher specific heat capacity than.
Show All Work And Units.
Answers q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. What does it mean if water has a higher specific heat capacity than oil? Show all work and proper. It requires more energy to raise its temperature by the same amount.
Use Q = (M)(Δt)(Cp) To Solve The Following Problems.
Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the.