Molality Worksheet With Answers Pdf - A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of ethanol. Chem 101 worksheet 7 dr. Calculate the molality of sucrose in a solution composed of 11.31 g of sucrose (c12h22o11) dissolved in 606 ml of water.
Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of ethanol. Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 11.31 g of sucrose (c12h22o11) dissolved in 606 ml of water. What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Chem 101 worksheet 7 dr. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh.
Calculate the molality of sucrose in a solution composed of 11.31 g of sucrose (c12h22o11) dissolved in 606 ml of water. What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of ethanol. Chem 101 worksheet 7 dr. A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution.
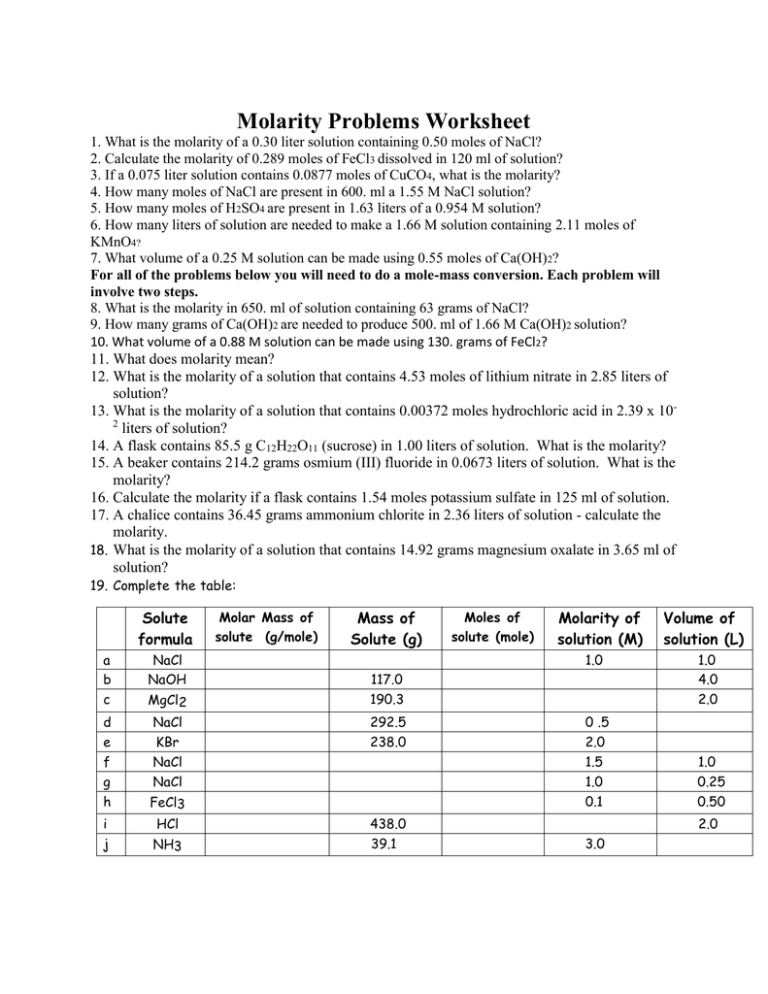
Molarity_Worksheet 1 ans key Molar Concentration Magnesium
A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of.
Molarity And Molality Worksheet
Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of ethanol. What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of.
Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key
Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 11.31 g of sucrose (c12h22o11) dissolved in 606 ml of water. Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into.
Molarity Worksheet 1 Answer Key Chemistry
Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. What is.
Molarity and Dilution Worksheets Download Free PDF Molar
A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of ethanol. Calculate the molality of sucrose in a solution composed of 11.31 g of sucrose (c12h22o11) dissolved in 606 ml of.
Molarity And Molality Worksheet prntbl.concejomunicipaldechinu.gov.co
Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml.
Molality and Molarity Worksheet PDF
Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into.
Molarity Practice Problems With Answers Molarity Problems Pr
A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Calculate the.
Molarity Molality Normality and Mass Percent Worksheet II.pdf Molar
What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Calculate the molality of sucrose in a solution composed of 11.31 g of sucrose (c12h22o11) dissolved in 606.
Molality Worksheet With Answers
What is the mole fraction of sodium chloride in a solution containing 0.23 moles of nacl and 5.5 moles of water? Chem 101 worksheet 7 dr. A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in.
Calculate The Molality Of Sucrose In A Solution Composed Of 11.31 G Of Sucrose (C12H22O11) Dissolved In 606 Ml Of Water.
Caddell problems 1.) what mass of magnesium nitrate, mg(no3)2, is needed to prepare 855 ml of a 0.575 m solution. Calculate the molality of sucrose in a solution composed of 12.36 g of sucrose (c12h22o11) dissolved in 636 ml of water. Calculate the molality of a solution prepared by dissolving 125 ml of pure methanol (density = 0.791 g/ml) into 275 g of ethanol. Chem 101 worksheet 7 dr.
What Is The Mole Fraction Of Sodium Chloride In A Solution Containing 0.23 Moles Of Nacl And 5.5 Moles Of Water?
A solution is prepared by mixing 100.0 g of water, h2o, and 100.0 g of ethanol, c2h5oh.