Intermolecular Forces Practice Worksheet - What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find it useful to.
You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide.
You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide.
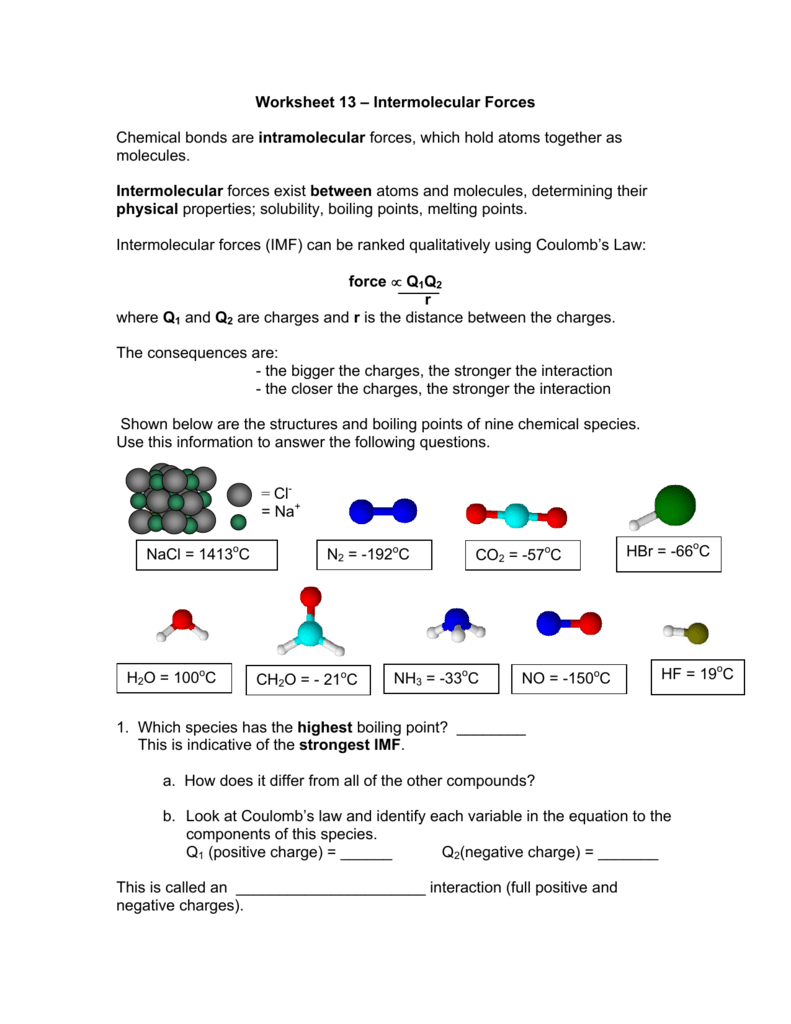
Worksheet 13 Intermolecular Forces Chemical bonds are
1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the.
Intermolecular Forces Worksheet And Answers Ch U4 A3 Intermo
1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. What is the strongest intermolecular force present for each of the following molecules? Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. You may find.
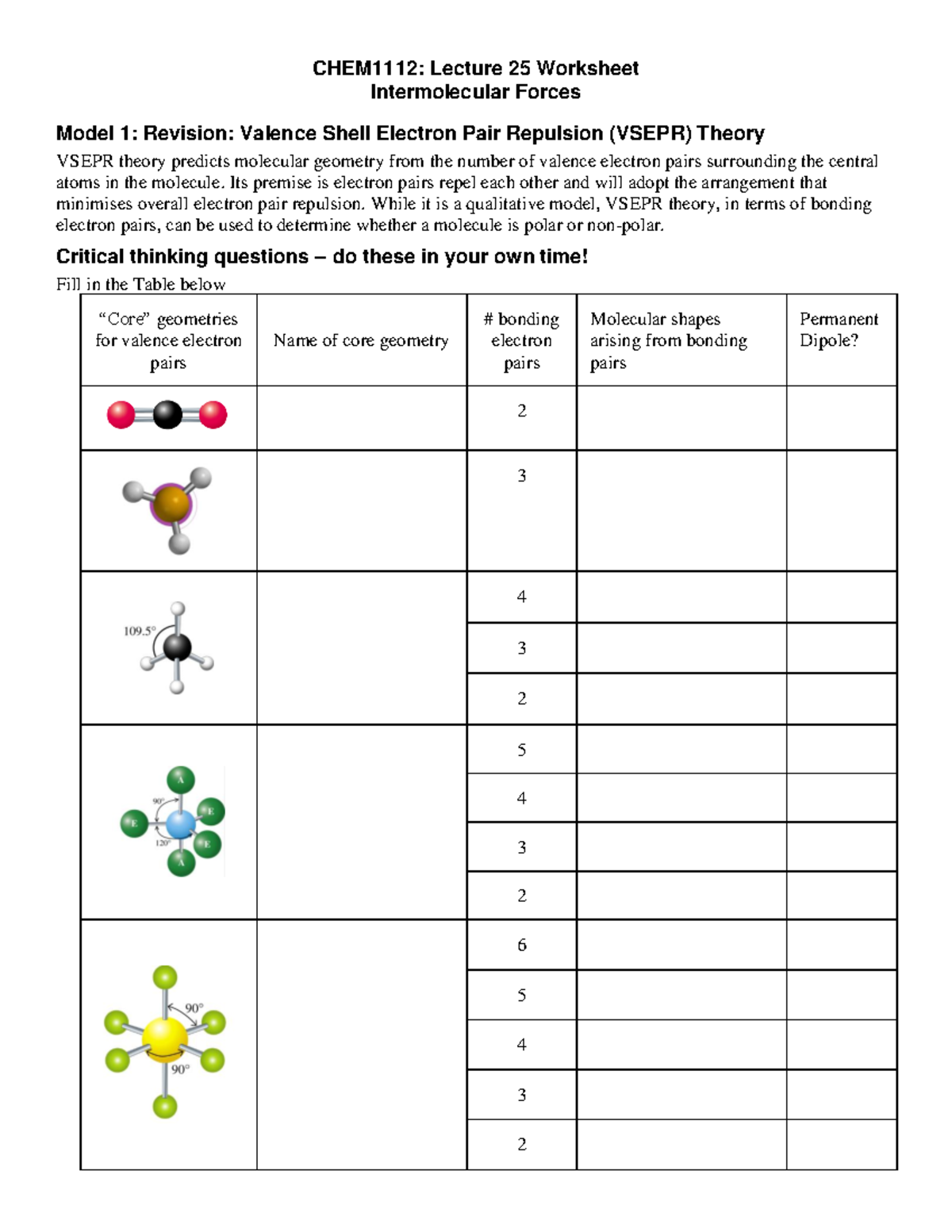
Worksheet 25 Lecture practice CHEM1112 Lecture 25 Worksheet
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the.
Intermolecular Forces Practice Worksheets
You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Intermolecular forces worksheet for each of the following compounds, determine the.
Intermolecular Forces Worksheet
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? Tell which member of each of the following pairs of compounds you would expect to have the.
Chemistry Intermolecular Forces Practice by Teach Simple
1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of.
Intermolecular Forces Worksheet Worksheet
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Intermolecular forces worksheet for each of the following compounds, determine the.
Intermolecular Forces Summary, Worksheet, And Key Worksheet
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to. What is the strongest intermolecular force present for each of.
3. intermolecular forces worksheet with key
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of the following molecules? You may find.
Chapter 10 Practice worksheets What type(s) of intermolecular
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces.
What Is The Strongest Intermolecular Force Present For Each Of The Following Molecules?
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to.