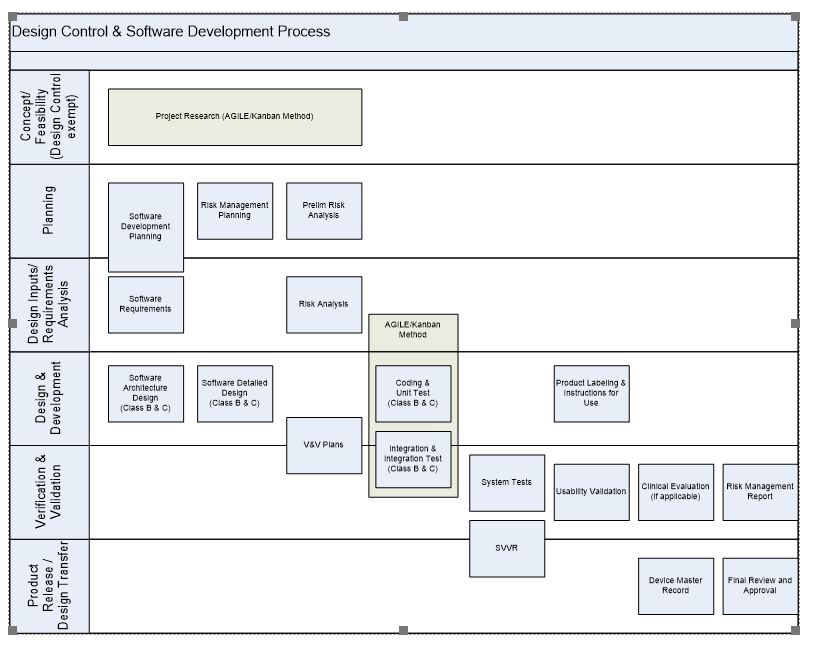
Iec 62304 Software Development Plan Template - Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. The iec 62304 describes how to develop and document software for medical devices. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. This is an overview over our free templates which. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Deliverables, traceability, regular update of. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. Sopproblemresolution • software development incl.
These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Deliverables, traceability, regular update of. This is an overview over our free templates which. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. The iec 62304 describes how to develop and document software for medical devices. Sopproblemresolution • software development incl. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution.
Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. Sopproblemresolution • software development incl. This is an overview over our free templates which. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. The iec 62304 describes how to develop and document software for medical devices. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. Deliverables, traceability, regular update of.
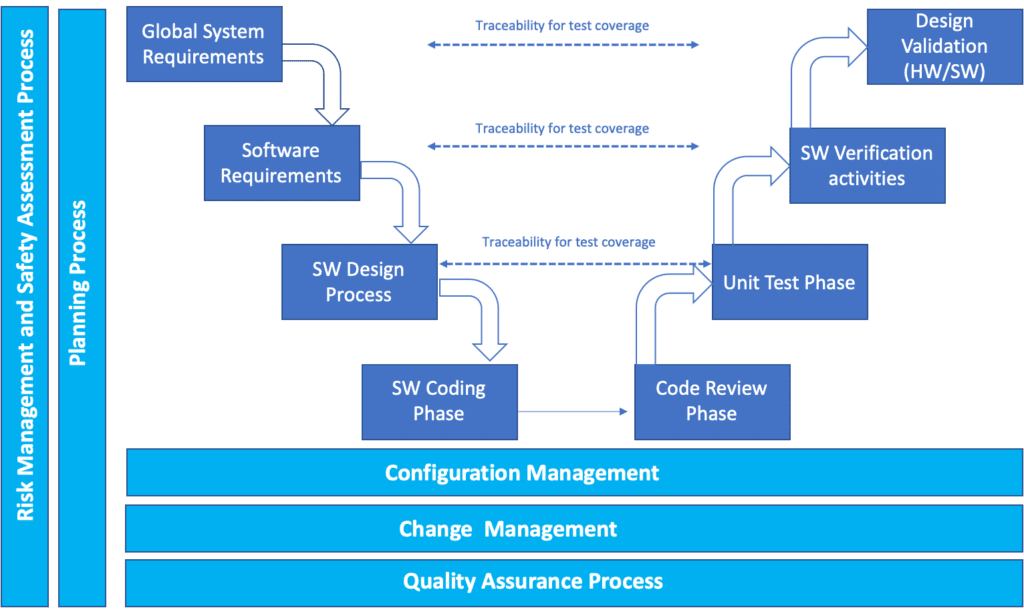
IEC 62304 Medical device Software life cycle
This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Deliverables, traceability, regular update of. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as..
Software Development Plan Template MedicalDeviceHQ
Sopproblemresolution • software development incl. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. This template.
IEC62304 Template Software Development Plan V1 0 PDF Software
These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Deliverables, traceability, regular update of. This is an overview over our free templates which. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Sopproblemresolution • software development incl.
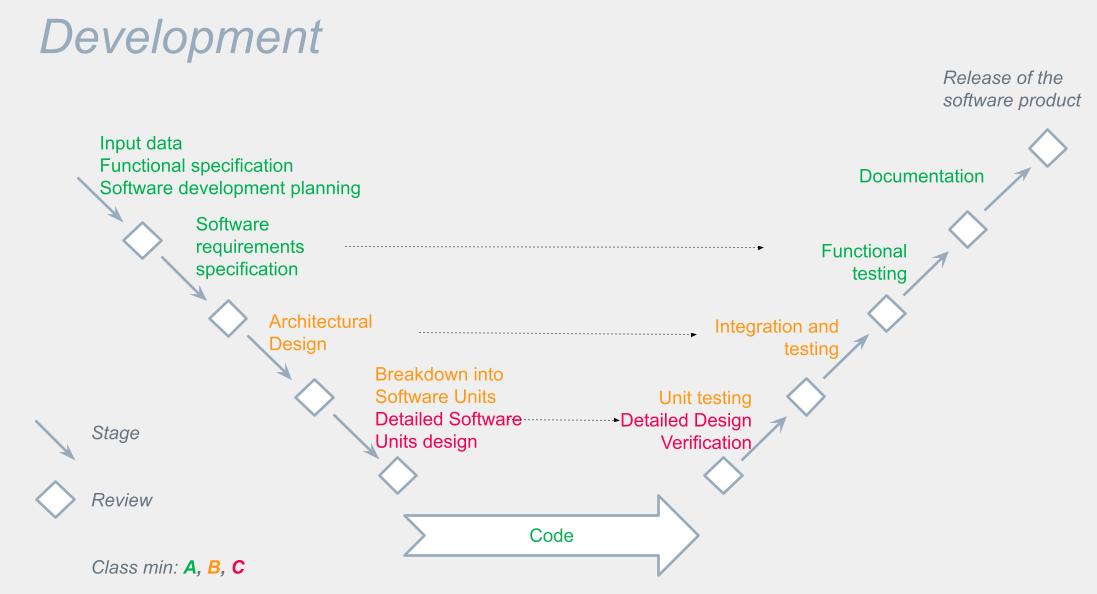
[PDF] Creation of an IEC 62304 compliant software development plan
The iec 62304 describes how to develop and document software for medical devices. This is an overview over our free templates which. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. Intland’s medical iec 62304.
Iec 62304 Software Development Plan Template
This is an overview over our free templates which. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. These templates deal with sections of iec 62304 about project.
Iec 62304 Software Development Plan Template
These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. This is an.
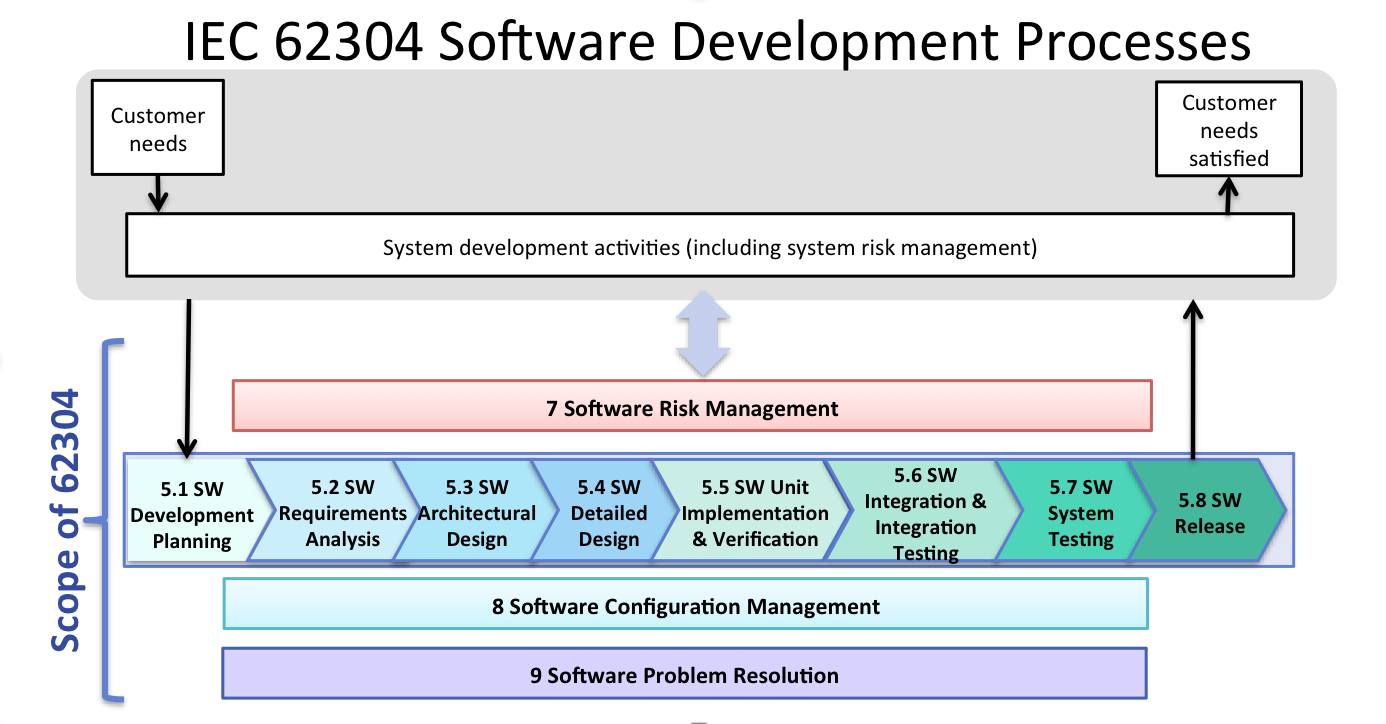
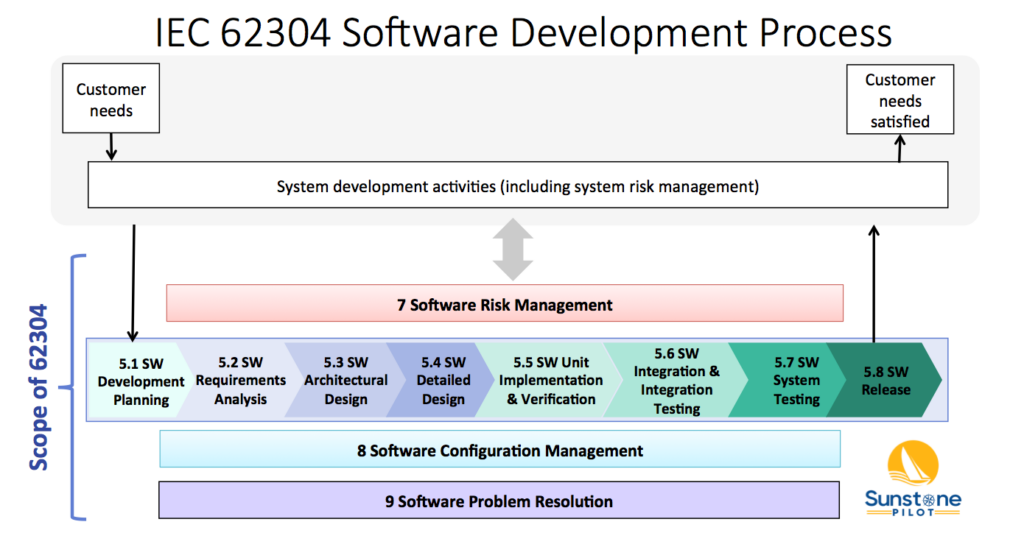
IEC 62304 Software Development Process Sunstone Pilot, Inc.
These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. The iec 62304 describes how to develop and document software for medical devices. Intland’s medical iec 62304 & iso 14971 template is.
FDA Software Guidances and the IEC 62304 Software Standard Sunstone
The iec 62304 describes how to develop and document software for medical devices. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. This template outlines the overall plan for software.
IEC62304 Template Software Development Plan PDF
Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Deliverables, traceability, regular update of. These forms can be just used as a checklist with notes taken separately for.
Iec 62304 Software Development Plan Template
These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. This is an overview over our free templates which. Deliverables, traceability, regular update of. Sopproblemresolution • software development incl.
Sopproblemresolution • Software Development Incl.
Deliverables, traceability, regular update of. This is an overview over our free templates which. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments.
Iec 62304 Software Safety Classification The Software Safety Classification For [Enter Device Name] Has Been Established As.
These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. The iec 62304 describes how to develop and document software for medical devices.


![[PDF] Creation of an IEC 62304 compliant software development plan](https://d3i71xaburhd42.cloudfront.net/52b435e220f8b1206dfa9d6fa34c19cef7a3135e/5-Table1-1.png)