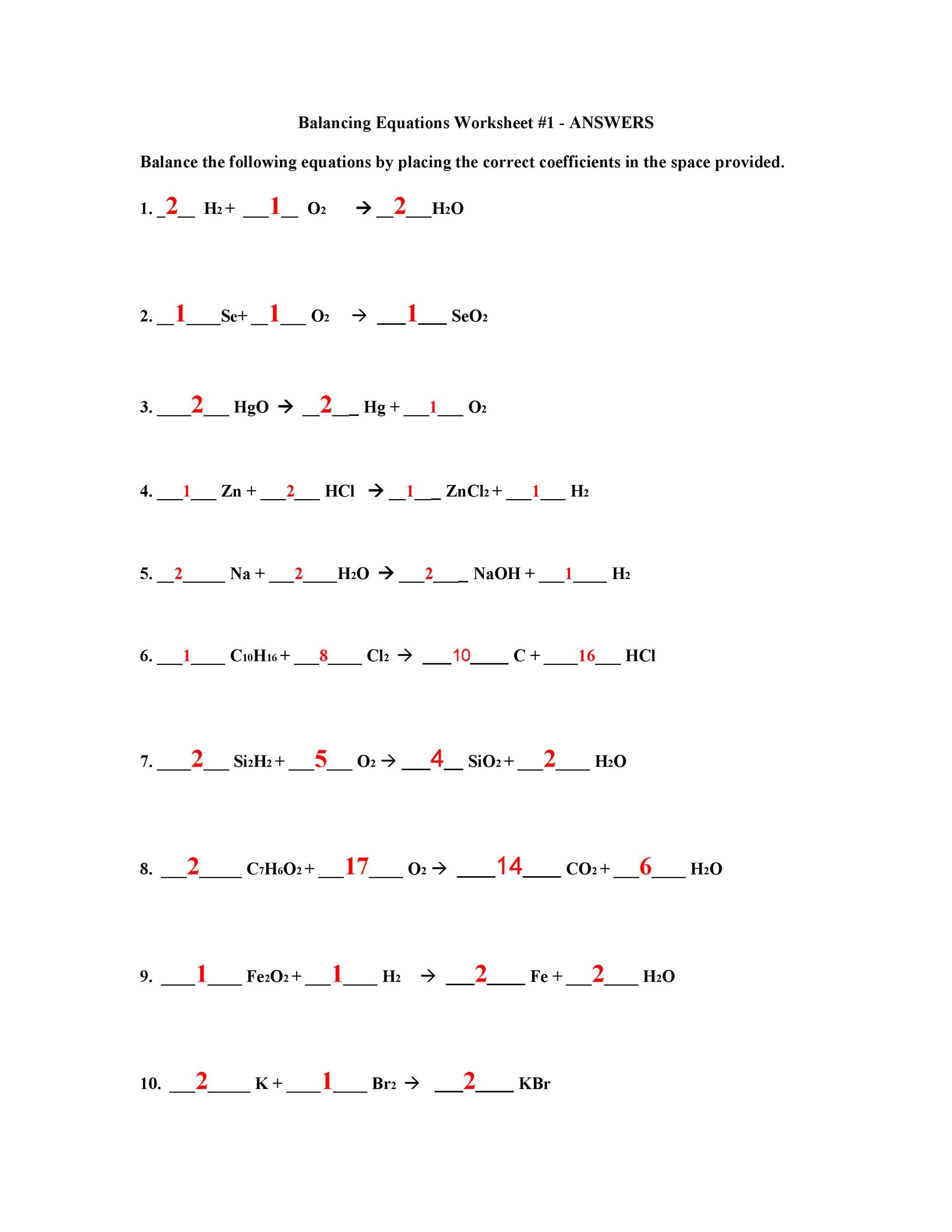
Balancing Equations Practice Worksheet Answer Key - Write the word equations below as chemical equations and balance: (coefficients equal to one (1) do not need to be shown in your answers). Balancing equations practice worksheet balance the following equations: 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead.
Write the word equations below as chemical equations and balance: 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. (coefficients equal to one (1) do not need to be shown in your answers). Balancing equations practice worksheet balance the following equations: 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead.
(coefficients equal to one (1) do not need to be shown in your answers). 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Balancing equations practice worksheet balance the following equations: 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. Write the word equations below as chemical equations and balance:
Balancing Chemical Equations Answers Key
Write the word equations below as chemical equations and balance: (coefficients equal to one (1) do not need to be shown in your answers). 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. Balancing.
Balancing Chemical Equations Practice With Answers Understan
Write the word equations below as chemical equations and balance: 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Balancing equations practice worksheet balance the following equations: (coefficients equal to one (1) do not.
Balancing Chemical Equations Worksheets With Answers
Write the word equations below as chemical equations and balance: 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. (coefficients equal to one (1) do not need to be shown in your answers). 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Balancing.
Introduction To Balancing Chemical Equations Worksheets
1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. Write the word equations below as chemical equations and balance: Balancing equations practice worksheet balance the following equations: (coefficients equal to one (1) do not need to be shown in your answers). 1) zinc and lead (ii) nitrate.
Balancing Chemical Equations Worksheet Answer Key 1 25 —
Balancing equations practice worksheet balance the following equations: (coefficients equal to one (1) do not need to be shown in your answers). 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Write the word.
Balancing Chemical Equations Answers Key
1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. (coefficients equal to one (1) do not need to be shown in your answers). Write the word equations below as chemical equations and balance: Balancing.
49 Balancing Chemical Equations Worksheets [with Answers]
(coefficients equal to one (1) do not need to be shown in your answers). Write the word equations below as chemical equations and balance: 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. Balancing.
Balancing Chemical Equations Worksheets 2 Answer Key
1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. Write the word equations below as chemical equations and balance: (coefficients equal to one (1) do not need to be shown in your answers). 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Balancing.
Balancing Chemical Equations Homework
(coefficients equal to one (1) do not need to be shown in your answers). 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. Balancing equations practice worksheet balance the following equations: Write the word.
Balancing Chemical Equations Easy Worksheet
Balancing equations practice worksheet balance the following equations: (coefficients equal to one (1) do not need to be shown in your answers). Write the word equations below as chemical equations and balance: 1) ___ nano 3 + ___ pbo ___ pb(no 3)2 + ___ na 2o 2) ___ agi + ___ fe 2(co 3)3. 1) zinc and lead (ii) nitrate.
1) ___ Nano 3 + ___ Pbo ___ Pb(No 3)2 + ___ Na 2O 2) ___ Agi + ___ Fe 2(Co 3)3.
Write the word equations below as chemical equations and balance: 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. (coefficients equal to one (1) do not need to be shown in your answers). Balancing equations practice worksheet balance the following equations:






![49 Balancing Chemical Equations Worksheets [with Answers]](http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-15.jpg)