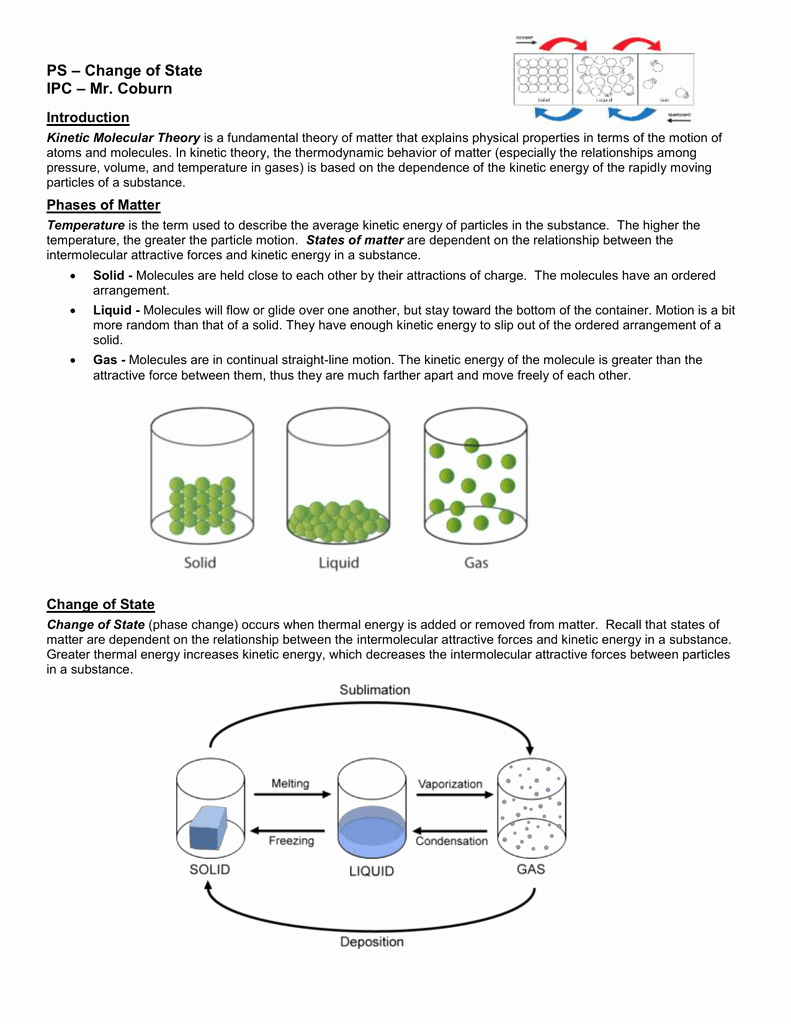
Answer Key Kinetic Molecular Theory Worksheet Answers - Ch 301 fall 2008 worksheet 10: Describe how gases, liquids, and solids compare using the following table. Intermolecular forces answer key 1. What do we mean when we say molecular view of. What is the major flaw with kinetic molecular theory that makes it unable to. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory.
Describe how gases, liquids, and solids compare using the following table. What is the major flaw with kinetic molecular theory that makes it unable to. Ch 301 fall 2008 worksheet 10: Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. What do we mean when we say molecular view of. Intermolecular forces answer key 1.
Describe how gases, liquids, and solids compare using the following table. Intermolecular forces answer key 1. Ch 301 fall 2008 worksheet 10: What do we mean when we say molecular view of. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. What is the major flaw with kinetic molecular theory that makes it unable to.
Molecular Theory Worksheet
Intermolecular forces answer key 1. What is the major flaw with kinetic molecular theory that makes it unable to. Describe how gases, liquids, and solids compare using the following table. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. Ch 301 fall 2008 worksheet 10:
Quiz & Worksheet Properties of Gases with the Molecular
What do we mean when we say molecular view of. Describe how gases, liquids, and solids compare using the following table. Intermolecular forces answer key 1. Ch 301 fall 2008 worksheet 10: Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory.
Molecular Theory Worksheet Answers Chemistry
Describe how gases, liquids, and solids compare using the following table. Ch 301 fall 2008 worksheet 10: What do we mean when we say molecular view of. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. Intermolecular forces answer key 1.
Answer Key Molecular Theory Worksheet Answers
Intermolecular forces answer key 1. Ch 301 fall 2008 worksheet 10: What do we mean when we say molecular view of. What is the major flaw with kinetic molecular theory that makes it unable to. Describe how gases, liquids, and solids compare using the following table.
Molecular Theory Worksheet Printable Word Searches
What do we mean when we say molecular view of. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. Ch 301 fall 2008 worksheet 10: What is the major flaw with kinetic molecular theory that makes it unable to. Describe how gases, liquids, and solids compare using the following table.
Molecular Theory Worksheet
Describe how gases, liquids, and solids compare using the following table. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. Ch 301 fall 2008 worksheet 10: What do we mean when we say molecular view of. What is the major flaw with kinetic molecular theory that makes it unable to.
Molecular Theory Worksheets Answer Key
Describe how gases, liquids, and solids compare using the following table. Ch 301 fall 2008 worksheet 10: Intermolecular forces answer key 1. What do we mean when we say molecular view of. What is the major flaw with kinetic molecular theory that makes it unable to.
Fillable Online Molecular Theory Worksheet Answers Fax Email
What do we mean when we say molecular view of. Ch 301 fall 2008 worksheet 10: Intermolecular forces answer key 1. Describe how gases, liquids, and solids compare using the following table. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory.
Answer Key Molecular Theory Worksheet Answers » Semanario
Describe how gases, liquids, and solids compare using the following table. What do we mean when we say molecular view of. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. What is the major flaw with kinetic molecular theory that makes it unable to. Ch 301 fall 2008 worksheet 10:
Molecular Theory Worksheet Answers Chemistry
What is the major flaw with kinetic molecular theory that makes it unable to. Describe how gases, liquids, and solids compare using the following table. Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. What do we mean when we say molecular view of. Ch 301 fall 2008 worksheet 10:
What Do We Mean When We Say Molecular View Of.
Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. Describe how gases, liquids, and solids compare using the following table. What is the major flaw with kinetic molecular theory that makes it unable to. Ch 301 fall 2008 worksheet 10: